Ecologically or Biologically Significant Areas (EBSAs)
The Marginal Ice Zone and the Seasonal Ice-Cover Over the Deep Arctic Ocean
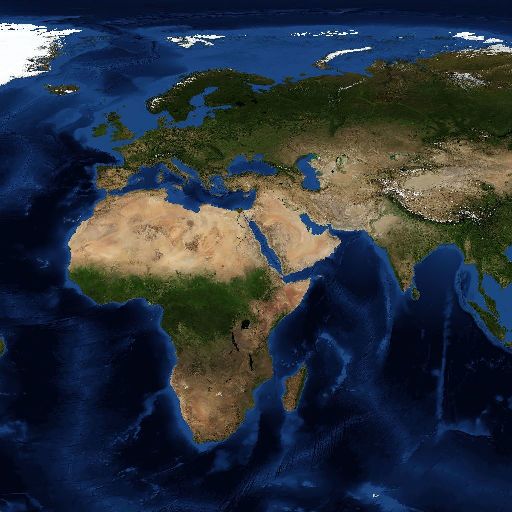
The marginal ice zone, which results from seasonal ice cover over the deeper (>500 m) parts of the Arctic Ocean, is a globally and regionally significant habitat and a unique feature of the area beyond national jurisdiction (figure 1). This type of habitat is found nowhere else in the Arctic.
The dramatic reduction of multi-year ice area means that large areas of the basins now have annual ice and are thus ice edges and seasonal ice zones with a period of open water in summer. This significant new region of ice edge/seasonal ice and seasonal open water over the deep Arctic is highly dynamic both spatially and temporally.
The previously very low biological production of the deep basins may change in this region as light, temperature and storminess increase and currents shift. In addition, wind-driven mixing of the ocean is more efficient over open water and over the thinner, more-mobile, seasonal ice than over multi-year ice, with the potential to increase productivity as well.
As in other areas of the Arctic, the marginal ice zone provides critical feeding habitat for a variety of icedependent species, including endangered species. Unlike the rest of the Arctic, however, the ice margin and the seasonal ice in the Central Arctic Ocean beyond national jurisdiction extend uniquely over deep water. This ice supports the majority of production in the stratified, low productivity waters of the region and plays a major role in contributing to the overall productivity of the region. See figure 2 for a conceptual model of the ecosystem at the marginal ice zone.
It is noted that, given the dynamic nature of the geographic area covered by this description, it may, depending on changes in coverage of multi-year ice/ marginal ice cover, overlap partially with an area meeting the CBD EBSA criteria that was described by the joint OSPAR/NEAFC/CBD workshop in the North-East Atlantic. Following peer review by the International Council for the Exploration of the Sea (ICES), the description of this area is currently under consideration by the Contracting Parties to OSPAR and NEAFC.
This area comprises the surface ice and related water column features associated with the marginal sea ice area in waters more than 500 m deep in areas beyond national jurisdiction. The marginal ice zone, at the edge of the ice pack, is a geographically and temporally dynamic feature and also changes in area, shape and geographic location from year to year, due to interannual variability of the Arctic ice pack. The multi-year marginal ice range of this area has been restricted to areas beyond national jurisdiction and waters greater than 500 m deep within the geographic scope of the workshop.
There is limited information about the ecosystems of the central Arctic Ocean. There is more literature describing the shallower, coastal areas of the Arctic (although these areas are also less studied than most shallow, coastal areas at lower latitudes). Where appropriate, this description includes some information
from coastal Arctic areas.
Production and lower trophic level communities
Ice algal communities can be divided into communities on the surface, interior and bottom of the ice (Horner et al. 1992). The surface can then be divided into melt-pond and infiltration communities, the interior into diffuse, brine-channel and band communities and the bottom into interstitial and sub-ice communities. All, except for the band community, occur in annual ice. In addition to microalgae, bacteria are an important component of the ice-algal community, but many other groups of organisms (e.g., archaea, fungi, ciliates,
kinetoplastids, choanoflagellates, amoebae, heliozoans, foraminiferans and some protists that belong to no
known group) also occur in these ice communities (Lizotte 2003). Poulin et al. (2010) reported a total of
1027 sympagic taxa in the Arctic (including in coastal waters).
There are known sampling biases in unicellular eucaryotes, by location (more coastal), size (more larger),
and season (Poulin et al. 2010), and these biases weaken or impede assessment of patterns and trends in
these taxa.
In general, there are steep gradients in temperature, salinity, light and nutrient concentrations, creating different habitats throughout the ice; the bottom 0.2 m has the most favourable conditions for growth among the interior communities (Arrigo 2003). However, with respect to biomass and contribution to primary production, the sub-ice community is the most important in the annual ice. In the outermost, thinnest part of the sea ice, phytoplankton occur predominantly in the sub-ice community, especially centric diatoms, in addition to a few colony-forming pennate diatoms. The sub-ice community of old annual ice is characterised by the pennate diatom, Nitzschia frigida, but other species, such as Nitzschia promare can be important locally (Syvertsen 1991). Melosira arctica (a species typical of multi-year ice) may dominate subice communities in some localities (von Quillfeldt et al. 2009). In addition there are seasonal trends and interannual variations in species composition, biomass and production as a result of several factors, among others, light, age and origin of the ice (e.g., distance to land and water depth). Thus, there is a high spatial
heterogeneity when larger areas are considered. All of these factors make it difficult to estimate regional
production (McMinn & Hegseth 2007).
Sea ice algae start to grow before phytoplankton. An extended growth season in Arctic areas forms ice algal
communities that are grazed actively by both ice fauna and zooplankton and may be an important component
of the diet of some species during the winter. Ice algae contribute 4 to 26% of total primary production in seasonally ice-covered waters (Gosselin et al. 1997, Sakshaug 2004). Apherusa glacial is probably the most numerous amphipod species in the central Arctic Ocean. Onisimus glacialis may be common in some areas.
The marginal ice zone is a highly productive area for phytoplankton (Sakshaug and Skjoldal 1989). Stable water masses due to sea-ice melt, coupled with high nutrient availability and light, result in an intense phytoplankton bloom. As water masses become stratified due to surface heating, nutrient flow from below is inhibited. Consequently, the bloom in marginal ice areas starts earlier than in areas never experiencing sea ice. The bloom follows the ice edge as it retreats in the spring. This “spring bloom” can occur in late August or even September in the areas of maximum ice retreat (Falk-Petersen et al. 2008). The ice-edge bloom is likely to weaken with time over the season (Wassmann et al. 2006). Arctic planktonic herbivores, such as Calanus hyperboreus, are able to utilize the vast area of the Arctic Ocean and to feed and store lipids for over-wintering until the sun disappears in October (Falk-Petersen et al. 2008). Calanus hyperboreus comprises up to half the zooplankton biomasses in the deep Arctic Ocean, and this is the only the Calanus speciesthat can remain established within the deep Arctic Ocean, (i.e., it can reproduce there) (Kosobokova 2012).
Fish
The fish diversity of the Arctic is described in the Arctic Biodiversity Assessment (Christiansen and Reist 2013, and literature quoted). The Arctic Central Basin has a disproportionately low taxa richness compared with the rest of the Arctic Ocean and adjacent sea regions, with only 13 species in four families and a proportion of Arctic species of around 92%. The number of species may be underestimated due to poor sampling, low abundances and unresolved taxonomy. Polar cod (Boreogadus saida), a keystone species in the marine Arctic, and ice cod (Arctogadus glacialis) are endemic to the Arctic and are the only fishes in the northern hemisphere that utilize sea ice as habitat and spawning substrate. Polar cod is the only marine fish species that is widespread throughout the entire Arctic Ocean and adjacent seas, including the Arctic Central Basin, i.e., it occurrs in areas with multi-year, annual sea ice and open water.
Ice cod is much less abundant and is primarily associated with fjords and Arctic shelves. Melnikov and Chernova (2013) assumed that the scale of the under-ice swarming polar cod in the Central Arctic (pack ice areas) is comparable to that observed in the ice-free areas at the Arctic periphery.
Birds
There are limited data on seabird distribution in the central Arctic Ocean. The following 13 seabird species make use of the deep Arctic Ocean for feeding: Northern fulmar (Fulmarus glacialis), Red phalarope (Phalaropus fulicarius), Parasitic skua (Stercorarius parasiticus), Pomarine skua (Stercorarius pomarinus), Glaucous gull (Larus hyperboreus), ivory gull (Pagophila eburnean), Kittiwake (Rissa tridactyla), Ross’s gull (Rodosthetia rosea), Sabine’s gull (Xema sabini), Arctic tern (Sterna paradise), Little auk (Alle alle), Black guillemot (Cepphus grille) and Brunnich's guillemot (Uria lomvia) (Buinitsky 1946, Portenko1946, Paynter 1955, Rutilevsky 1957, Uspensky 1968, Blomqvist & Elander 1987, Parmelee & Parmelee 1994, Vuilleumier 1996, Hjort et al. 1997, Lunk & Joern 2007, Gilg et al. 2010a,b).
Among them, the most common is Ross’s gull, which migrates post-breeding to feed on crustaceans in the
pack ice of the Arctic Ocean on a regular base (Blomquist & Elander 1981, Hjort et al. 1997, Gavrilo, unpublished). Ivory gulls prefer to use the marginal ice zone (Gilg et al. 2010, Gavrilo, unpublished). Figures 3 to 5 show observations of ivory gull, Ross’s gull and black guillemot.
Mammals
Ringed seal
The Arctic ringed seal Pusa (Phoca) hispida has a very large population size and broad distribution in the Arctic Ocean. Figure 6 shows encounters in the central Arctic Ocean. Ringed seals use sea ice exclusively for breeding, moulting and resting (haul-out), and feed on small schooling fish and invertebrates. In a co-evolution with one of their main predators, the polar bear, they developed the ability to create and maintain breathing holes in relatively thick ice, which makes them well adapted to living in ice covered waters. Kovacs et al. (2008) document declines in population size of this subspecies in parts of its range associated with a decrease in sea ice, and there are concerns that future changes in Arctic sea ice will have a similar negative impacts.
Polar bear
Polar bears (Ursus maritimus) are dependent on sea ice and are therefore particularly vulnerable to changes in sea ice extent, duration and thickness. Their circumpolar distribution, with 19 subpopulations, is limited by the southern extent of sea ice (Gorbunov & Belikov 2008). Figure 7 shows encounters in the central Arctic Ocean. In the summer, a great many of these subpopulations inhabit Arctic seas and use the marginal ice zone as an important feeding ground. In the winter, the polar bears are distributed more evenly throughout the Arctic ice, however with the highest abundance in areas with polynyas and leads. Preferred prey species of the polar bear are ringed seal and bearded seal, and in some areas harp seal.
Narwhal
Narwhals (Monodon monoceros) occur primarily in Arctic waters connected to the North Atlantic Ocean
(Reeves et al. 2014). It is a highly ice-dependent species that could make use of the central Arctic Ocean,
but there is no documented information on its distribution in these deeper waters. Narwhals are deepdiving
benthic feeders and forage on fish, squid and shrimp, especially Arctic fish species, such as Greenland halibut, Arctic cod and polar cod at up to 1500 m depth and mostly in winter. A recent assessment of the sensitivity of all Arctic marine mammals to climate change ranked the narwhal as one of the three most sensitive species, primarily due to its narrow geographic distribution, specialized feeding and habitat choice, and high site fidelity (Laidre et al. 2008 in Jefferson et al. 2008).
Beluga
Belugas (Delphinapterus leucas) are an Arctic species that have been tracked using this area at the edge of a range that is predominantly over the shallower Chuchki and Beaufort seas off North America (Hauser et al. 2014). Luque and Ferguson (2010), although not explicitly examining belugas from this area, note that populations of belugas at higher latitudes have a larger body size than those further south.
Bowhead whale
Bowhead whale (Balaena mysticetus) is the third of the three ice-associated cetacean species that reside year-round in the Arctic, mostly connected to the marginal ice zone. So far there are no observations of
this (heavily depleted) species in the central Arctic Ocean. The distribution of bowhead whales is nearly circumpolar, although the heavy ice conditions that have prevailed over the last millennium in the Arctic Basin have impeded (but not completely blocked) their movement in the Northwest and Northeast Passages. Some populations of bowhead whales are increasing (Reeves et al. 2014, and literature quoted).
Replacement of thick, multi-year ice by thin, first-year ice as the Arctic warms may contribute to increases in
the frequencies and magnitude of ice algal and phytoplankton blooms (Post et al. 2013).
Primary production of sea ice algae plays a crucial role in the life cycle of planktonic and benthic organisms (Gradinger 1995) in the Arctic Ocean, but the extent of this importance in annual ice in the deeper central Arctic Ocean has not been studied. However, a widespread deposition of ice algal biomass of on average 9 g
C per m2 to the deep-sea floor of the Arctic Central Basin has been observed (Boetius et al. 2013). When released from sea ice, ice algae may be an early (and only) seasonal food source for zooplankton. Thus, possible consequences of the observed thinning of the Arctic sea ice may be severe. If the sea ice disappears there will be a shift from a system dependent on sea ice species towards a system dependent on
phytoplankton species.
A change in timing and duration of the ice edge bloom increases the probability of a “mismatch” in productivity, which may have severe consequences for zooplankton that are dependent on this bloom today, with potential cascading effects throughout the ecosystem. However, the timing of ice formation and melt also influences the distribution and intensity of the primary production in the water column. Such primary production is likely to increase in areas with less sea ice but may then become limited by nutrient availability. The extent of nutrient replenishment by vertical mixing during winter is especially important for the level of productivity in ice-free waters (Smetacek & Nicol 2005). Thus, changed ice conditions may affect the productivity over the deep ocean of the Arctic more severely than shelf areas.
Of the observed increase in annual primary production in the Arctic from 2006 to 2007, 30% was attributable to decreased minimum summer ice extent and 70% to a longer phytoplankton growing season (Arrigo et al. 2008). On the other hand, reduced sea ice cover coupled with an an increase in atmospheric low pressures cells (with more wind) may cause the upper mixing layer to deepen and in turn causes changes in the relative importance of the algal groups that dominate the phytoplankton community. It has been suggested that mixing in the upper layers (above 40 m) favours diatoms (i.e., areas often influenced by sea ice), mixing down to 60-80 m favours Phaocystis pouchetii, while mixing below 80 m favours small nanoflagellates (Sakshaug 2004). However, increased stratification (due to melting sea ice and river input) and nutrient depletion in the euphotic zone may cause shifts in the taxonomic composition of phytoplankton (Tremblay et al. 2012), as recently recorded by increasing abundances of small-sized (<2 μm in diameter) phytoplankton cells (Li et al. 2009).Thus, the quality of the food available for grazing communities will most probably change. The importance of the ice edge related production for higher predators will change, but may depend on other factors, for example seabirds may be also be influenced by distance from breeding colonies.
Arndt C.E. & Swadling K.M. 2006. Crustacea in Arctic and Antarctic sea ice: distribution, diet and life
history strategies. Adv. Mar. Biol. 51, 197-315.
Arrigo K.R. 2003. Primary production in sea ice. In D.N. Thomas & G.S. Dieckmann (eds.): Sea ice. An
introduction to its physics, chemistry, biology and geology. pp. 143-183. Oxford: Blackwell
Publishing.
Arrigo K.R., van Dijken G. & Pabi S. 2008. Impact of a shrinking Arctic ice cover on marine primary
production. Geophysical Research Letters 35(19), L19603.
Blomquist S. & Elander M. 1981. Sabine’s gull (Xema sabini), Ross’s gull (Rhodostethia rosea) and
ivory gull (Pagophila eburnea). Gulls in the Arctic: A review. Arctic 34,122–132.
Bluhm B.A., Gebruk A.V., Gradinger R., Hopcroft R.R., Huettmann F., Kosobokova K.N., Sirenko B.I.
& Weslawski J.M. 2011. Arctic marine biodiversity: An update of species richness and examples
of biodiversity change. Oceanography 24(3), 232-240.
Boetius A., Albrect S., Bakker K., Bienhold C., Felden J., Fernandez-Mendez M., Hendricks S., Katlein
C., Lalande C., Krumpen T., Nicolaus M., Peeken I., Rabe B., Rogacheva A., Rybakova E.
Somavilla R., Wenzhofer F. & SC, R.V.P.A.-,- S. 2013. Export of algal biomass from the melting
Arctic sea ice. Science (126), 1430-1432.
Buinitsky V.K. 1946. Birds and mammals observation logbook. Proceedings of drifting expedition of
Glavsevmorput on ice-breaking steamer Georgy Sedov in 1937-1940. Moscow-Leningrad, 3,
5-13.
CAFF 2010. Arctic Biodiversity Trends 2010 – Selected indicators of change. CAFF International
Secretariat, Akureyri, Iceland. May 2010. Page 59.
Christiansen J.S. & Reist J.D. (lead authors) 2013. Chapter 6. Fishes. pp. 193-245 in Meltofte, H. (ed.)
Arctic Biodiversity Assessment. Status and trends in Arctic biodiversity. Conservation of Arctic
Flora and Fauna, Akureyri.
Eamer J., Donaldson G.M., Gaston A.J., Kosobokova K.N., Lárusson K.F., Melnikov I.A., Reist J.D.,
Richardson E., Staples L. & von Quillfeldt C.H. 2013. Life Linked to Ice: A guide to sea-iceassociated
biodiversity in this time of rapid change. CAFF Assessment Series No. 10.
Conservation of Arctic Flora and Fauna, Iceland. ISBN: 978-9935-431-25-7.
Falk-Petersen S., Leu E., Berge J., Kwasniewski S., Nygård H, Røstad A., Keskinen E., Thormar J., von
Quillfeldt C., Wold A. & Gulliksen B. 2008. Vertical migration in high Arctic waters during
autumn 2004. Deep-Sea Research II 55, 2275-2284.
Gilg O., Strøm H., Aebischer A., Gavrilo M.V., Volkov A.E., Miljeteig C. & Sabard S. 2010. Postbreeding
movements of northeast Atlantic ivory gull Pagophila eburnea populations – J. Avian
Biol. 41 (5), 532–542, doi: 10.1111/j.1600-048X.2010.05125.x
Gilg O., Strøm H., Gavrilo M.V., Boertmann D., Wiebe H. & Aebischer A. 2010. The ivory gull: a new
flagship species to study the impact of climate change in Arctic Seas? Arctic Frontiers–2010.
Living in the Far North. Unpublished presentation.
Gorbunov Y.A. & Belikov S.E. 2008. Observations of marine mammals and polar bear in the Arctic
Basin. Marine mammals of the Holarctic. Collection of scientific papers after the fifth
International Conference, Odessa, Ukraine, October 14-18, 2008. Odessa. pp. 220-222.
Gosselin M., Levasseur M., Wheeler P.A., Horner R.A. & Booth B.C. 1997. New measurements of
phytoplankton and ice algal production in the Arctic Ocean. Deep-Sea Res. 44, 1623-1644.
Gradinger R. 1995. Climate change and biological oceanography of the Arctic Ocean. Philosophical
Transactions of the Royal Society of London Series A-Mathematical Physical and Engineering
Sciences 352 (1699), 277-286.
Hauser D.D.W., Laidre K.L., Moore S.E., Suydam R.S. & Richard P.R. 2014. Diving behaviour and
distribution of beluga whales across diverse habitats of the Pacific Arctic. Presentation made
available to workshop.
Hjort C., Gudmundsson G.A. & Elander M. 1997. Ross’s gulls in the Central Arctic Ocean. Arctic 50(4),
289 – 292.
Horner R., Ackley S.F., Dieckmann G.S., Gulliksen B., Hoshiai T., Melnikov I.A., Reeburgh W.S.,
Spindler M. & Sullivan C.W. 1992. Ecology of Sea Ice Biota. 1. Habitat and terminology. Polar
Biology 12, 417–427.
IPCC 2013. Summary for Policymakers. In: Climate Change 2013: The Physical Science Basis.
Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel
on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung,
A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge,
United Kingdom and New York, NY, USA.
JeffersonT.A., Karczmarski L., Laidre K., O’Corry-Crowe G., Reeves R.R., Rojas-Bracho L., Secchi
E.R., Slooten E., Smith B.D., Wang J.R. & Zhou K. 2008. Monodon monoceros. In IUCN 2011.
IUCN Red List of threatened Species. Version 2011. 1. www.iucnredlist.org Downloaded on 31
August 2011.
Kosobokova K.N. 2012. Zooplankton of the Arctic Basin: community structure, ecology, distribution
patterns. Moscow, GEOS 271 pp.
Kovacs K., Lowry L. & HärkönenT. 2008. Pusa hispida. In IUCN 2011. IUCN Red List of Threatened
Species. Version 2011.1. www.iucnredlist.org. Downloaded on 31 August 2011.
Li W.K.W., McLaughlin F.A., Lovejoy C. & Carmack E.C. 2009. Smallest algae thrive as the Arctic
Ocean freshens. Science 326, 539-539.
Lizotte M.P. 2003. The microbiology of sea ice. In D.N. Thomas & G.S. Dieckmann, (eds.) Sea ice: An
introduction to its physics, chemistry, biology and geology. pp. 184-210. Oxford: Blackwell
Publishing.
Lunk S. & Joern D. 2007. Ornithological observations in the Barents and Kara Seas during the summers
of 2003, 2004 and 2005. Russian Ornithological Journal. Express issue N, 377 pp.
Luque S.P. & Ferguson S.H. 2010. Age structure, growth, mortality, and density of belugas
(Delphinapterus leucas) in the Canadian Arctic: responses to environment? Polar Biology 33,
163-178.
McMinn A. & Hegseth E.N. 2007. Sea ice primary productivity in the northern Barents Sea, spring 2004.
Polar Biology 30(3), 289-294.
Melnikov I.A. & Chernova N.V. 2013. Characteristics of Under-Ice Swarming of Polar Cod Boreogadus
saida (Gadidae) in the Central Arctic Ocean. Journal of Ichthyology, 2013, Vol. 53, No. 1,
pp. 7-15. © Pleiades Publishing, Ltd., 2013. Original Russian Text © I.A. Melnikov, N.V.
Chernova, 2013, published in Voprosy Ikhtiologii, 2013, Vol. 53, No. 1, pp. 22–30.
Meltofte H. (ed.) 2013. Arctic Biodiversity Assessment. Status and trends in Arctic biodiversity.
Conservation of Arctic Flora and Fauna, Akureyri.
Parmelee D.F. & Parmellee J.M. 1994. Bird sightings from a nuclear-powered ice breaker from across the
Arctic Ocean to the geographic North Pole 90”N.-Ornithologists. - Wilson Bull., 106(2), 391-392.
Paynter R.A. Jr. 1955. Birds in the Upper Arctic. Auk. 72, 79 – 80.
Portenko L.A. 1946. Birds of high latitudes of the Arctic Ocean. Proceedings of drifting expedition of
Glavsevmorput on ice-breaking steamer Georgy Sedov in 1937-1940. Moscow-Leningrad, Vol. 3,
19-29.
Post E., Bhatt U.S., Bitz C.M., Brodie J.F., Fulton T.L., Hebblewhite M., Kerby J., Kutz S.J., Stirling I. &
Walker I. 2013. Ecological consequences of sea-ice decline. Science 341, 519-524.
Poulin M., Daugbjerg N., Gradinger R., Ilyash L., Ratkova T. & von Quillfeldt C.H. 2010. The pan-Arctic
biodiversity of marine pelagic and sea-ice unicellular eukaryotes: A first-attempt assessment
Marine Biodiversity. Marine Biodiversity 41, 13-28.
Reeves R.R, Ewins P.J., Agbayani S., Heide-Jørgensen M.P., Kovacs K.M., Lydersen C., Suydam R.,
Elliott W., Polet G., van Dijk Y, & Blijleven R. 2014. Distribution of endemic cetaceans in
relation to hydrocarbon development and commercial shipping in a warming Arctic. Marine
Policy 44, 375-389.
Rudels B., Larsson A.-M. & Sehlstcdt P.-I. 1991. Stratification and water mass formation in the Arctic
Ocean: some implications for the nutrient distribution. pp. 19-31 in Sakshaug. E., Hopkins. C. C.
E. & Oritsland. N. A. (eds.): Proceedings of the Pro Mare Symposium on Polar Marine Ecology,
Trondhcim. 12-16 May 1YW. Polar Research 10(1), 19-31.
Rutilevsky G.L. 1957. Fauna of mammals and birds of the Central Arctic (based on observations of
drifting stations). Proc. of the AARI.Moscow 205, 5–18.
Sakshaug E. 2004. Primary and secondary production in the Arctic Sea. pp. 57-81 in Stein R. &
Macdonald R.W. (eds). The organic carbon cycle in the Arctic Ocean. Springer, Berlin.
Sakshaug E. & Skjoldal H.R. 1989. Life at the ice edge. Ambio 18, 60-67.
Slooten E., Smith B.D., Wang J.Y. & Zhou K. 2008. Monodon monoceros. In IUCN 2011. IUCN Red
List of Threatened Species. Version 2011.1. www.iucnredlist.org. Downloaded on 31 August
2011.
Smetacek V. & Nicol S. 2005. Polar ocean ecosystems in a changing world. Nature 437, 362-368.
Syvertsen E.E. 1991. Ice algae in the Barents Sea: types of assemblages, origin, fate and role in the
ice-edge phytoplankton bloom. Polar Research 10(1), 277-288.
Tremblay J.-E., Robert D., Varela D., Lovejoy C., Darnis G., Nelson R.J., Sastri A. 2012. Current state
and trends in Canadian Arctic marine ecosystems: I Primary production. Climate Change DOI
10.1007/s10584-012-0496-3.
Uspensky S.M. 1968. Life in the High latitudes. Birds as an example. Moscow. 464 pp.
Vongraven D. & Peacock E. 2011. Development of a pan-Arctic monitoring plan for polar bears:
background paper. Circumpolar Biodiversity Monitoring Programme, CAFF Monitoring Series
Report No.1, January 2011, CAFF International Secretariat, Akureyri, Iceland. ISBN 978-9935-
431-01-1.
von Quillfeldt C.H., Hegseth E.N., Johnsen G., Sakshaug E. & Syvertsen E.E. 2009. Ice algae.
pp. 285-302 in Sakshaug E., Johnsen G. & Kovacs K. (eds) Ecosystem Barents Sea. Tapir
Academic Press, Trondheim, Norway.
Vuilleumier F. 1996. Birds observed in the Arctic Ocean to the North Pole. Arctic and Alpine Research
28 (1): 18 – 122.
Wassmann P., Reigstad M., Haug T. Rudels B., Carroll M.L., Hop H., Gabrielsen G.W., Falk-Petersen S.,
Denisenko S.G., Arashkevich E., Slagstad D. & Pavlova O. 2006a. Food webs and carbon flux in
the Barents Sea. Progress in Oceanography 71(2-4), 232-287.
Werner I. & Gradinger R. 2002. Under-ice amphipods in the Greenland Sea and Fram Strait (Arctic):
environmental controls and seasonal patterns below the pack ice. Marine Biology 140(2),
317-326.
- Additional_figures_ARC_1.pdf (/api/v2013/documents/95700FFB-A8F7-2887-827E-914ACDB23674/attachments/Additional_figures_ARC_1.pdf)
- ARC_1_EBSA-GIS shapefile.zip (/api/v2013/documents/95700FFB-A8F7-2887-827E-914ACDB23674/attachments/ARC_1_EBSA-GIS%20shapefile.zip)
- dec-COP-12-DEC-22
The area is unique because the marginal ice and associated seasonal ice occurs over a deep ocean basin. Hence the dynamics of its nutrient supply are globally unique, with implications for the primary production in the area (Rudels et al. 1991). In addition, the importance of ice algae as a pathway of productivity into the food web (Gradinger 1995, Gosselin et al. 1997, Sakshaug 2004) is unique at least within the Northern hemisphere.
Important for ice-dependent species such as polar cod (Christiansen and Reist 2013), ringed seal (Kovacs et al. 2008), polar bear (Gorbunov & Belikov 2008), possibly narwhal, Ross’s gull (Blomquist & Elander 1981, Hjort et al. 1997, Gavrilo, unpublished) and ivory gull (Gilg et al. 2010, Gavrilo, unpublished).
The marginal ice zone is particularly important as a feeding ground for seals, polar bears and ivory gulls due to its enhanced productivity.
Calanus hyperboreus comprises up to half the zooplankton biomass in the deep Arctic Ocean and is the only Calanus species that can remain established within the deep Arctic Ocean (i.e.; it can reproduce there) (Kosobokova 2012).
Polar bear (IUCN vulnerable) (Gorbunov & Belikov 2008,Vongraven &Peacock 2011) and ivory gull (IUCN near threatened) (Gilg et al. 2010, Gavrilo, unpublished) depend on the sea ice throughout their life cycles.
The geographical extent of the seasonal ice cover is declining in the summer (IPCC 2013)
Ice algae constitutes the second source of primary production in Arctic seas, with the highest relative contribution in the central Arctic Ocean (Gosselin et al. 1997). Increasing extent of annually formed sea ice over the Arctic Ocean, with vanishing and restricted multi-year ice limited to the northern regions of the Canadian Archipelago and Greenland (as reported for 2008 by the US National Snow and Ice Centre), may result in higher biomass of sympagic unicellular eukaryote taxa available for the upper trophic levels at the time of minimum irradiance reaching the polar surface waters (Poulin et al. 2010).
Productivity of both ice algae (Gosselin et al. 1997, Sakshaug 2004) and phytoplankton (Sakshaug & Skjoldal 1989) is higher in the marginal ice zone than in the more open waters, and deeper into the centre of the ice pack, so the marginal ice zone scores high on productivity relative to other areas of the Arctic.
In addition to microalgae, bacteria are an important component of the ice-algal community, but many other groups of organisms (e.g., archaea, fungi, ciliates, kinetoplastids, choanoflagellates, amoebae, heliozoans, foraminiferans, some protists that belong to no known group, Rotifera, Nematoda, Copepoda, Amphipoda) also occur in ice communities (Werner & Gradinger 2002, Lizotte 2003, Arndt & Swadling 2006, Bluhm et al. 2011, Kosobokova 2012). Consequently, biodiversity of the lower trophic levels in the ice is relatively high.
Very low impact from human activities (but vulnerable for climate change, which is already acting) (Meltofte et al. 2013, Eamer et al. 2013).